Molecular weight (also known as molar mass) is a fundamental concept in chemistry that represents the mass of one mole of a substance. Whether you’re a student working on chemistry homework, a researcher conducting experiments, or a professional in the pharmaceutical industry, accurately calculating molecular weight is essential for various scientific applications. This comprehensive guide explains what molecular weight is, how to calculate it, and why it matters across different fields.
What Is Molecular Weight?

Molecular weight, also called molecular mass or molar mass, is the sum of the atomic weights of all atoms in a molecule. It represents the mass of one mole of a substance and is typically expressed in grams per mole (g/mol). Understanding molecular weight is crucial for stoichiometry calculations, solution preparation, and many other chemical applications.
The molecular mass of a compound is calculated by adding together the atomic masses of each constituent atom in the molecule. For example, to calculate the molecular weight of water (H₂O), you would add the atomic mass of two hydrogen atoms and one oxygen atom.
Need to Calculate Molecular Weight?
Save time and ensure accuracy with our easy-to-use molecular weight calculator tool.
Understanding the Terminology: Molecular Mass vs. Molar Mass
Molecular Mass
Molecular mass (or molecular weight) is the mass of one molecule of a substance expressed in unified atomic mass units (u). One unified atomic mass unit (u) is defined as 1/12 the mass of one atom of carbon-12.
Molar Mass
Molar mass is the mass of one mole of a substance expressed in grams per mole (g/mol). One mole contains exactly 6.02214076 × 10²³ molecules (Avogadro’s number).

While these terms are often used interchangeably, they have distinct meanings. The key difference is that molecular mass refers to a single molecule, while molar mass refers to one mole of the substance. However, their numerical values are the same, just expressed in different units (u for molecular mass and g/mol for molar mass).
How to Calculate Molecular Weight
Calculating the molecular weight of a compound involves a few simple steps:
Example Calculation: Water (H₂O)
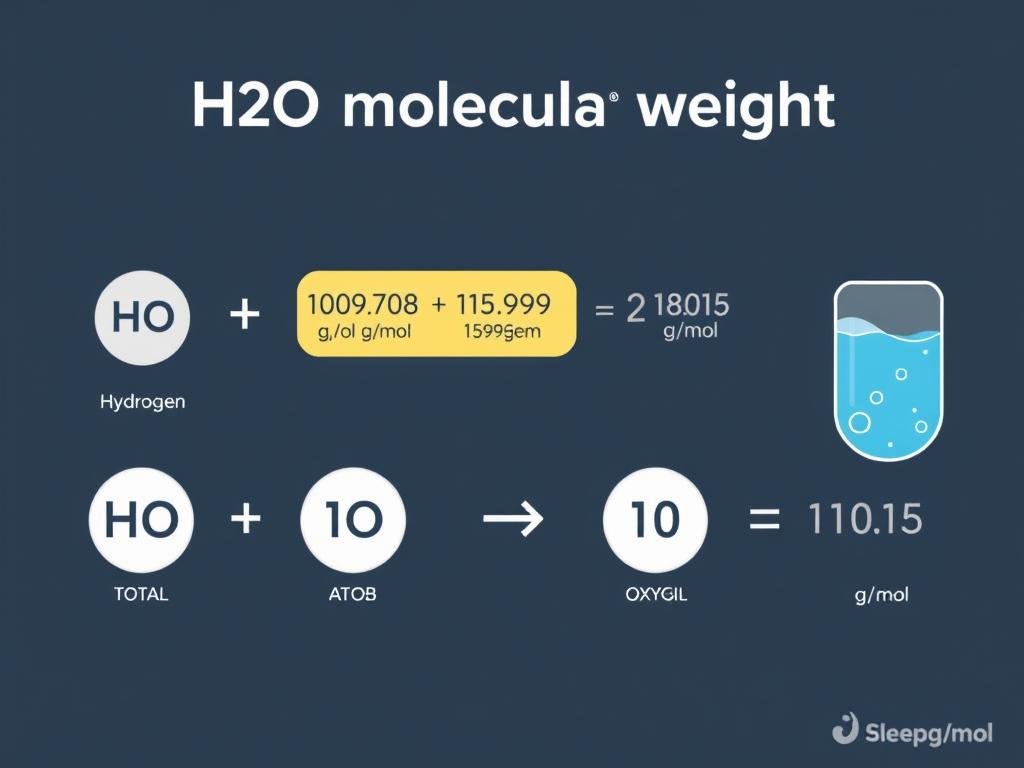
- Hydrogen (H): 1.008 g/mol × 2 atoms = 2.016 g/mol
- Oxygen (O): 15.999 g/mol × 1 atom = 15.999 g/mol
- Total molecular weight of H₂O = 2.016 + 15.999 = 18.015 g/mol
Step-by-Step Calculation
Example Calculation: Glucose (C₆H₁₂O₆)
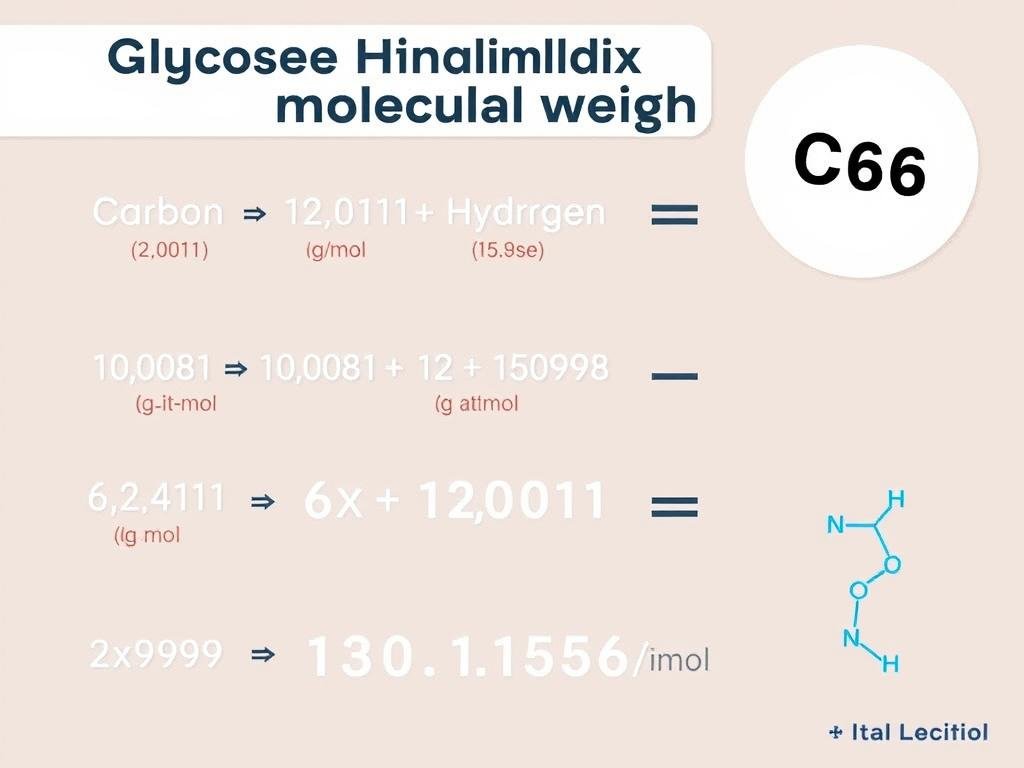
- Carbon (C): 12.011 g/mol × 6 atoms = 72.066 g/mol
- Hydrogen (H): 1.008 g/mol × 12 atoms = 12.096 g/mol
- Oxygen (O): 15.999 g/mol × 6 atoms = 95.994 g/mol
- Total molecular weight of C₆H₁₂O₆ = 72.066 + 12.096 + 95.994 = 180.156 g/mol
Step-by-Step Calculation
Skip the Manual Calculations
Our molecular weight calculator handles complex formulas instantly, saving you time and preventing errors.
Importance and Applications of Molecular Weight
Understanding molecular weight is essential across various scientific disciplines and industries:
Chemistry

- Solution preparation and concentration calculations
- Stoichiometry in chemical reactions
- Analysis of chemical compounds
Biology and Biochemistry

- Protein and DNA analysis
- Enzyme kinetics studies
- Cell metabolism research
Pharmaceutical Industry

- Drug discovery and development
- Quality control of medications
- Pharmacokinetics studies
Additional Applications
Environmental Science
Molecular weight calculations help in analyzing pollutants, understanding environmental processes, and developing remediation strategies. For instance, when studying the movement of contaminants in groundwater, molecular weight affects how substances dissolve and migrate.

Materials Science
In materials science and engineering, molecular weight is crucial for understanding polymer properties, developing new materials, and quality control. The molecular weight of polymers directly influences their physical properties such as strength, flexibility, and melting point.

Common Challenges in Molecular Weight Calculations
Complex Chemical Formulas
For compounds with complex formulas, manual calculation becomes time-consuming and prone to errors. This is especially true for large biomolecules like proteins or polymers with repeating units.

Isotopic Variations
Elements can exist as different isotopes with varying atomic masses. Standard molecular weight calculations typically use average atomic masses, but in some applications (like mass spectrometry), isotopic distributions must be considered.

Hydrates and Mixed Compounds
Compounds that include water molecules (hydrates) or exist as mixtures require special consideration when calculating molecular weight. For example, copper(II) sulfate pentahydrate (CuSO₄·5H₂O) includes five water molecules in its formula.
Overcome Calculation Challenges
Our molecular weight calculator handles complex formulas, isotopes, and special cases with ease.
Practical Examples and Applications
Solution Preparation in Laboratory Settings
When preparing solutions of specific concentrations, molecular weight is essential for accurate calculations. For example, to prepare a 1 molar (1M) solution of sodium chloride (NaCl), you need to know its molecular weight (58.44 g/mol) to determine that 58.44 grams should be dissolved in enough water to make 1 liter of solution.

Drug Dosage Calculations
In pharmaceutical applications, molecular weight is crucial for determining drug dosages and understanding drug interactions. The molecular weight of a drug affects its absorption, distribution, metabolism, and excretion in the body.
Research Applications
In research settings, molecular weight determination is often a critical step in compound identification and characterization. Techniques like mass spectrometry rely on molecular weight to identify unknown compounds or verify the purity of synthesized materials.

| Field | Application | Example Calculation |
| Chemistry | Solution preparation | To make 500 mL of 0.1M NaOH, you need 0.1 mol/L × 40.00 g/mol × 0.5 L = 2 g NaOH |
| Biochemistry | Protein analysis | A 150 amino acid protein has an average amino acid MW of 110 g/mol, giving an approximate MW of 16,500 g/mol |
| Pharmacy | Drug formulation | Converting 10 mg of a drug salt (MW 350 g/mol) to its free base (MW 300 g/mol): 10 mg × (300/350) = 8.57 mg |
| Environmental | Pollutant analysis | Converting 5 ppm of carbon dioxide (CO₂, MW 44.01 g/mol) to mg/m³: 5 ppm × 44.01/24.45 = 9 mg/m³ at STP |
Why Use a Molecular Weight Calculator?
While manual calculations are possible for simple compounds, using a molecular weight calculator offers several advantages:
Benefits of Using a Calculator
- Saves time, especially for complex formulas
- Reduces the risk of calculation errors
- Handles special cases like hydrates and isotopes
- Provides consistent results for repeated calculations
- Allows for quick comparison of different compounds
Limitations to Be Aware Of
- Requires correct formula input
- May use average atomic masses by default
- Special notations might need interpretation
- Understanding the underlying principles is still important
Whether you’re a student learning chemistry, a researcher conducting experiments, or a professional in the pharmaceutical or chemical industry, a molecular weight calculator is an invaluable tool for accurate and efficient calculations.
Ready to Calculate Molecular Weights?
Our user-friendly calculator handles everything from simple compounds to complex biomolecules with precision and ease.